Speaking of Aether, or ether, as it is now written, is a pretty interesting thing. In metaphysical terms, it was deemed by Aristotle as the quintessence, the fifth element. It was the thing that the universe above the terrestrial earth was made of. It was where the gods lived and what they breathed. It's the kind of thing that one could imagine everything else sort of popping out of. Like words.
Tonight, I was working on my research project, and I had finally found a paper on Perkins condensations that I could access (without paying 30 pounds), and it turns out that I don't really know if it was what I was looking for. The language of the paper is so dense and complex that it's hard for me to pinpoint anything useful. Thus, I'm going to have to tell Dr Mullins tomorrow that I'm at a bit of an impasse, as it took me two hours just to find that one paper. Maybe he can point me in the right direction. Or else fail me in research. One of those.
Earlier in the day, I went to visit Dr Masingale, who I'm tutoring General Chemistry for. At one point, he asked me a question that dealt with density, but I couldn't think of the word "density" for some reason. That's when he handed me a plastic container of water, and then another container, a smaller one. As he handed it to me, he said, "Careful. Unless you've been lifting weights, you're going to have a hard time with this one!" And it was true, because the liquid inside of the bottle was Mercury. I don't know how he ended up with so much Mercury, but he told me he "accumulated it over time." I got so excited to be holding this bottle of this element I'd never seen for myself, to experience for myself how dense it really was. I really wanted to open the bottle and pour some out onto my hand and play with it. Then I remembered that mercury poisoning is no fun.
I also had my first workshop session today, for Organic. I think it went pretty well, although I've noticed that I'm still really awkward when it comes to public speaking. I don't think I'll ever be totally okay with it. I always just start panicking a little, and shove it down, because I know that I have to get to friggin' work. One question was really weird, though, because it involved Ca3N2, which I've never heard of before. When I looked up the structure, I was really surprised:
This is what it looks like. Just know that Ca and N should NOT be forming double bonds with each other, because of their difference in metallic character, etc. I want to know why it happens. Of course. I always want to know why weird things happen. I think that's why I liked Inorganic a lot; I learned that two metals, Cesium and Gold can form an ionic bond, even though they shouldn't. These are the kinds of things that keep me tied to something. I'm just curious.
I think I'll talk to Mullins about this Calcium-Nitrogen structure tomorrow, as long as he doesn't fire me as his research student. But that's unreasonable, right? I can't expected to find all the information forever, right?
P.S.
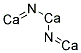

No comments:
Post a Comment